Our goal is to understand the sex-specific influence of androgens and estrogens on mitochondria in the healthy and diseased brain.
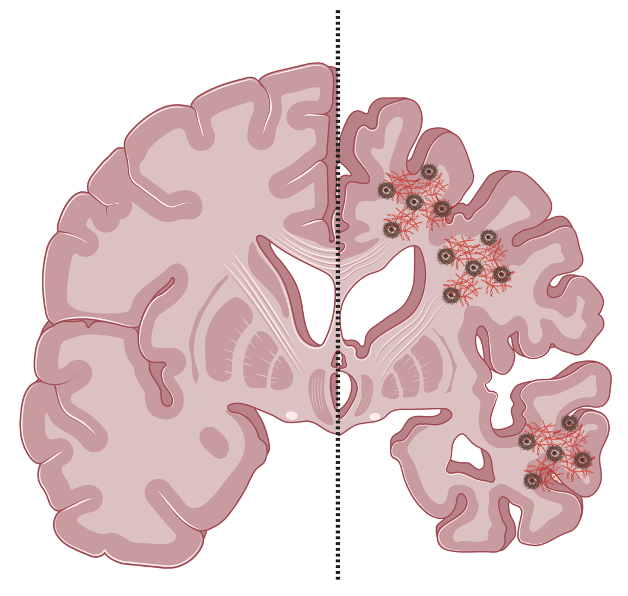
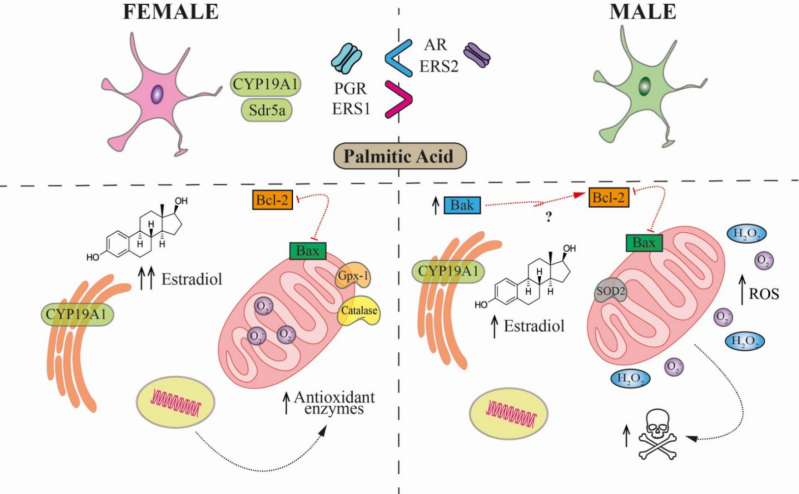
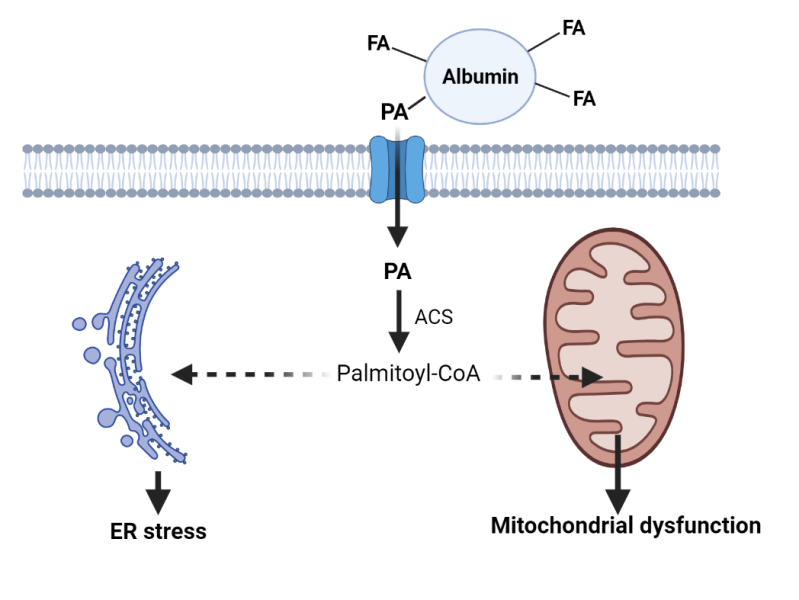
The interaction between sex hormones and brain mitochondria is a complex area of study with significant implications for understanding sex-specific differences in brain health and disease. Mitochondria, known as the powerhouse of the cell for their role in generating adenosine triphosphate (ATP), are involved in various functions beyond energy production, including calcium buffering, reactive oxygen species (ROS) regulation, apoptosis, and critical signalling pathways for neuronal survival and synaptic plasticity. Traditionally associated with reproductive processes and sexual characteristics, these hormones also play essential roles in neuroprotection, cognition, learning, and mood regulation. Their levels fluctuate and decrease across the lifespan, which is important for understanding the higher incidence and prevalence of neurodegenerative diseases (e.g. Alzheimer's disease) in women.
Understanding how sex hormones regulate mitochondrial dynamics in both healthy and diseased brains is essential to uncovering the biological mechanisms that drive sex differences in brain aging and vulnerability to neurodegeneration. By elucidating the interplay between hormones and mitochondria, our team aims to identify novel therapeutic targets to counteract mitochondrial dysfunction - a key contributor to age-related cognitive decline. Our work has revealed critical pathways through which hormonal signalling influences brain resilience or susceptibility, ultimately guiding the development of sex-specific interventions to promote healthy brain aging and reduce the burden of neurodegenerative diseases.
Fig. 1- Flowchart illustrating our overarching aims and research interests.