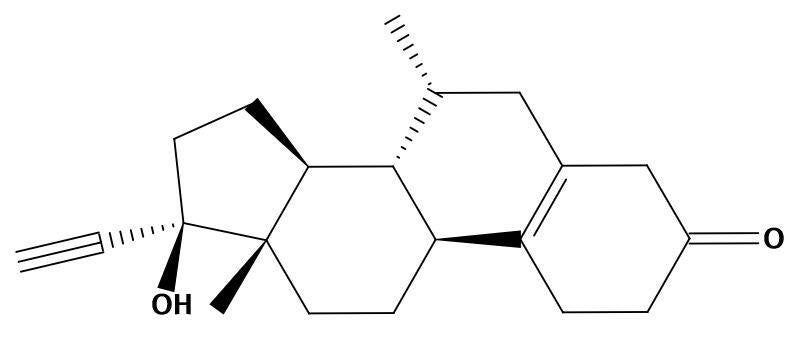
Aging is a complex biological process that involves changes in various physiological systems, including the endocrine and the nervous system. One of the factors that influence aging is hormonal decline, which occurs in both men and women, but more sharply in women after menopause. Hormonal decline can affect the function of various organs and tissues, including the brain, where it can increase the risk of developing neurodegenerative diseases such as Alzheimer’s disease.
One of the possible pathways by which hormonal decline can affect the brain is through the modulation of mitochondrial function. Mitochondria are organelles responsible for cellular energy production and for the control of oxidative stress, apoptosis and intracellular signaling. Mitochondrial dysfunction is one of the main mechanisms involved in the pathogenesis of Alzheimer’s, as it compromises the energy and redox homeostasis of the brain, favors the formation of toxic protein aggregates and induces inflammation and neuronal death.
Hormones can interact with mitochondria in various ways, modulating their biogenesis, dynamics, metabolism, respiration and stress response. Some studies suggest that sex hormones, such as estrogen and testosterone, can exert neuroprotective effects by improving mitochondrial function and reducing oxidative stress in the brain. However, the molecular and cellular mechanisms by which hormones regulate mitochondria are not fully elucidated, as well as the effects of hormonal decline on mitochondrial function during endocrine aging and menopause. Our goal is to fill this knowledge gap, using a multidisciplinary approach that combines experimental models in silico, in vitro and in vivo, as well as clinical samples from patients with different hormonal and cognitive profiles.
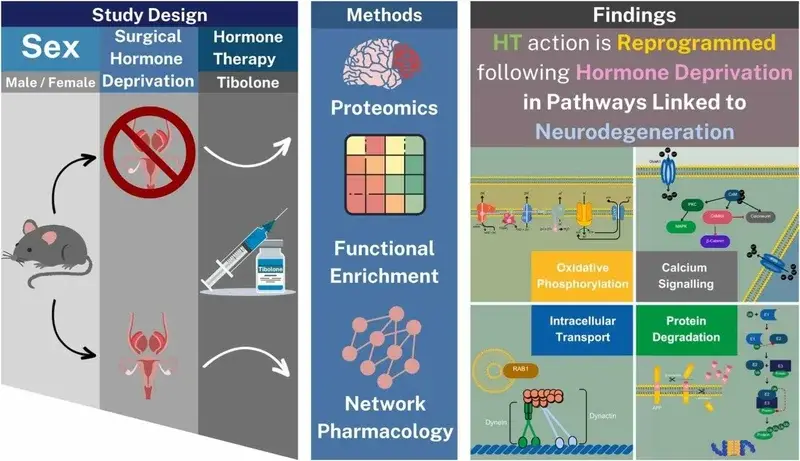
Both sex and gonadal hormone deficiency are strongly associated with neurodegeneration (McGovern et al., 2024).