Our research employs diverse cellular and molecular techniques to explore how hormonal regulation impacts signalling pathways critical for mitochondrial function and integrity. Given that mitochondrial protein expression and levels differ between sexes, it is essential to assess these responses in both males and females to drive precision medicine forward. To accomplish this, we conduct in-depth proteomic and transcriptomic profiling of astrocytes and whole-brain homogenates from both sexes, providing valuable insights into how sex and hormone interactions influence mitochondrial responses under neurodegenerative conditions. This approach is a vital step toward developing more precise and timely pharmacotherapies that account for sex-specific mitochondrial dynamics
Tibolone and testosterone preserve cell morphology and mitochondrial content, respectively, in astrocytic cells under glucose deprivation.
One of the major milestones of our group was the discovery that tibolone, a synthetic steroid drug used by women to alleviate symptoms associated with menopause, has the ability to upregulate mitochondrial proteins involved in transport and oxidative phosphorylation. Our group has previously reported that tibolone preserves mitochondrial membrane potential, reduces oxidative stress, and improves cell survival following metabolic dysfunction in glial cells. In fact, our previous studies have shown that tibolone has anti-inflammatory, antioxidant, and anti-apoptotic properties, making it a promising candidate for repurposing in neurometabolic diseases, including neurodegenerative diseases.
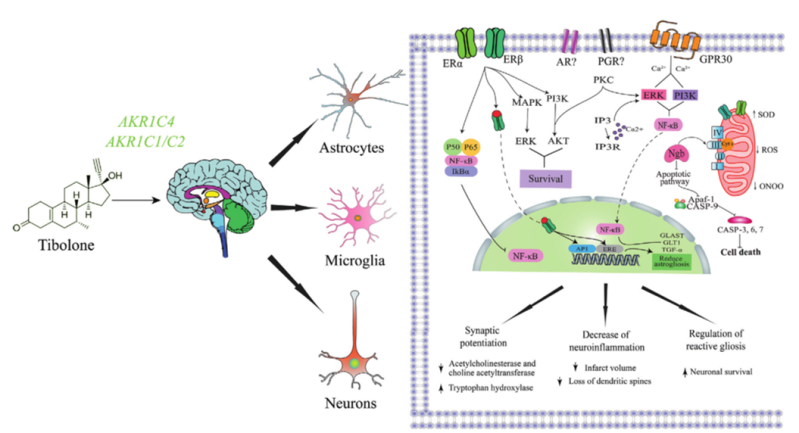
Tibolone exerts neuroprotective actions mediated by estrogen receptors (Del Río et al., 2020).

Sex differences in human astrocytes exposed to palmitic acid.
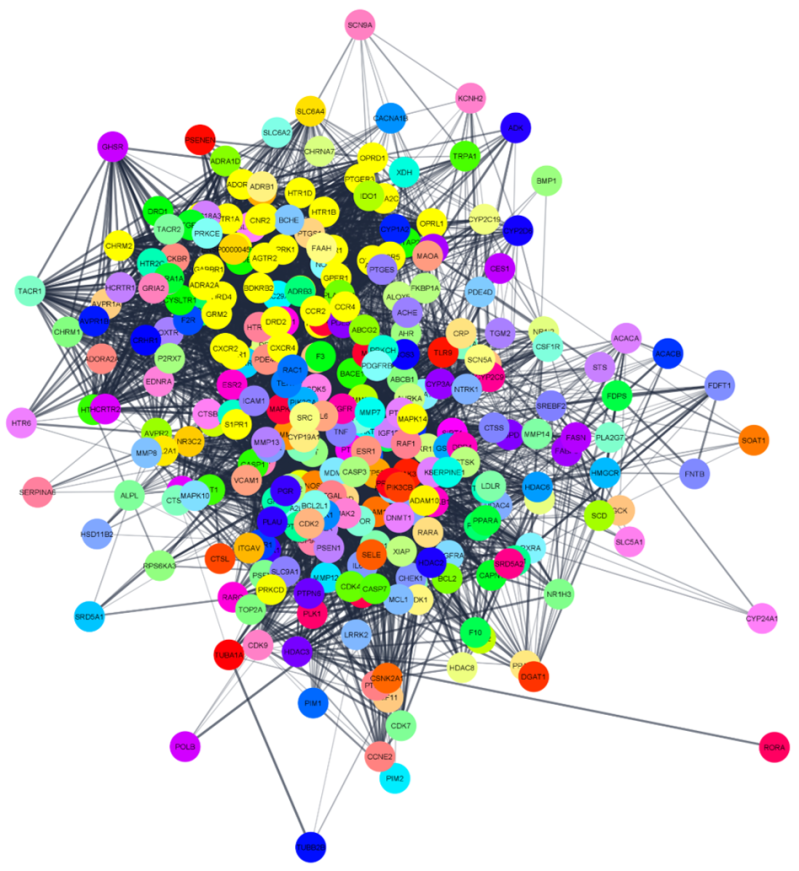
By leveraging network pharmacology to analyze protein-protein interaction networks, we can systematically identify and map druggable cellular targets involved in neurodegenerative diseases. This approach not only highlights individual proteins that may be therapeutically targeted but also reveals critical hubs - key proteins or molecular complexes that act as central nodes in these networks. Such hubs often drive disease mechanisms, and targeting them can have a substantial impact on disease progression. Network pharmacology provides an integrated view of these molecular interactions, enabling us to pinpoint novel therapeutic avenues and design multi-target drugs that modulate complex pathological pathways more effectively. This comprehensive approach opens new opportunities for precision therapies that can address the multifaceted nature of neurodegenerative disorders.
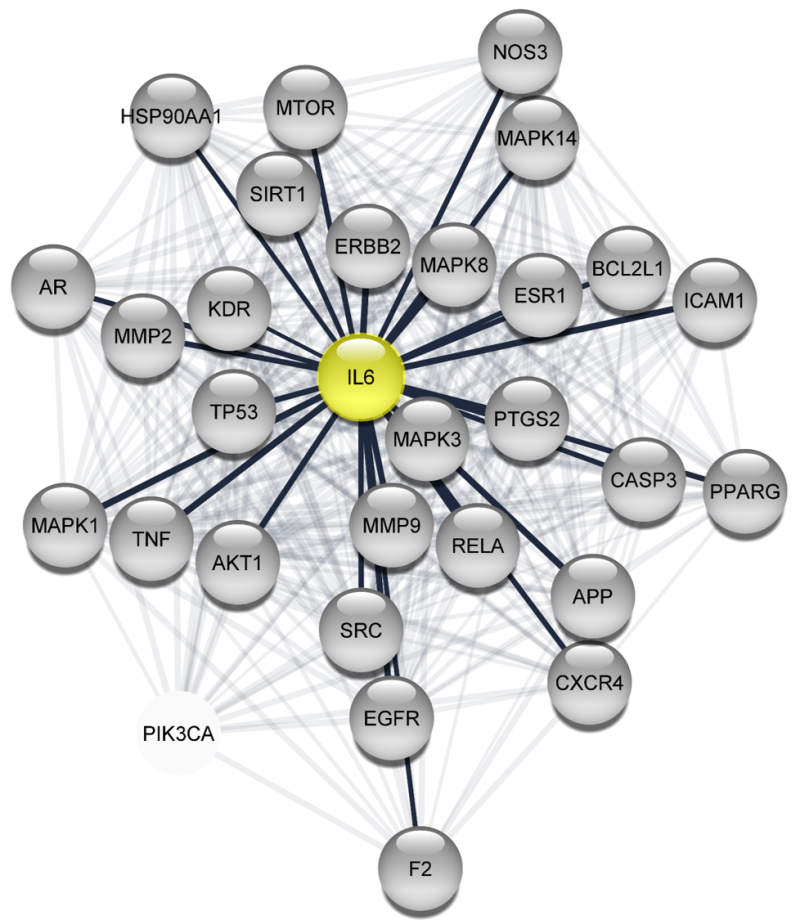
Interleukin-6 (IL6) as a hub regulated by tibolone and involved in traumatic brain injury pathology (McGovern and Barreto, 2021).

